Estimated read time: 3-4 minutes
This archived news story is available only for your personal, non-commercial use. Information in the story may be outdated or superseded by additional information. Reading or replaying the story in its archived form does not constitute a republication of the story.
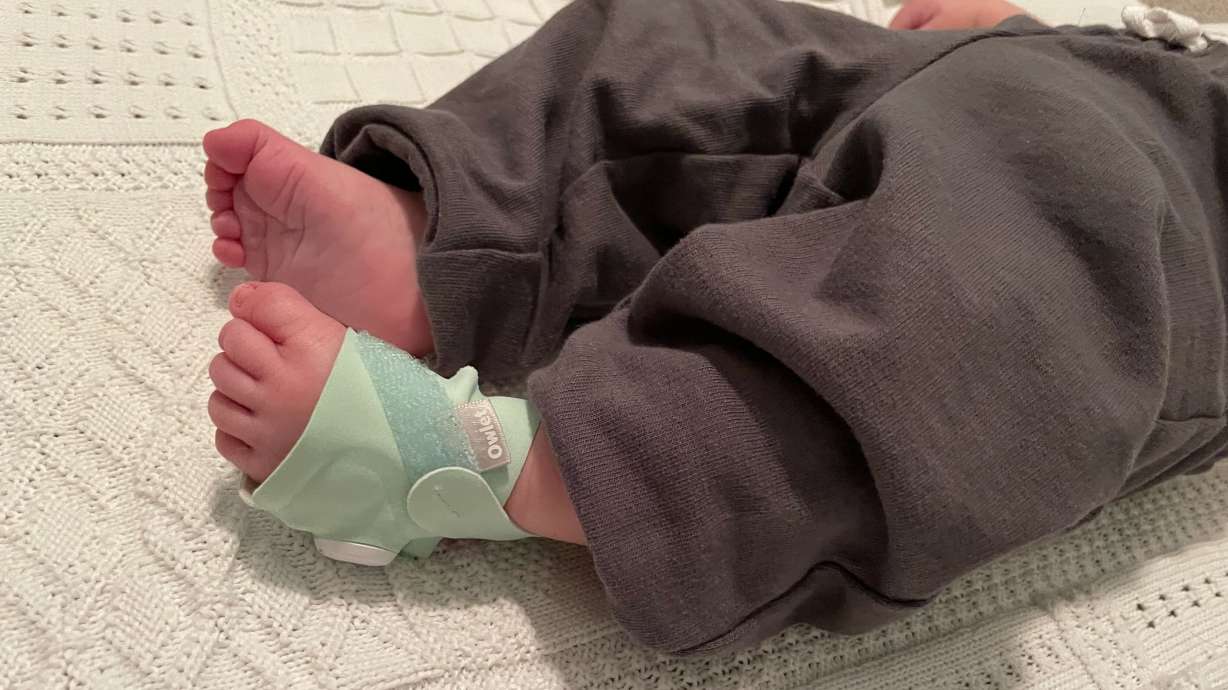
LEHI — Utah-based baby care tech company Owlet has received clearance from the Food and Drug Administration for its baby monitor Dream Sock.
Owlet announced the Dream Sock received de novo clearance from the FDA on Thursday, which means it will become the first over-the-counter medical-grade pulse oximetry device.
"Today marks a significant breakthrough in our journey to bring care to the home and empower parents with an unprecedented FDA-clearance for the Owlet Dream Sock," Owlet CEO Kurt Workman said. "This accomplishment not only signifies our commitment to innovation in the infant health category but, more importantly, our dedication to ensuring the health and well-being of every baby."
The Dream Sock monitors movement, wakings and live health readings including pulse rate and oxygen saturation level for infants 1 to 18 months old. The monitor will alert caregivers with lights and sound if the baby's health readings fall outside of preset ranges.
Owlet said in a press release the new, expanded medical-grade features will be added to all existing and new Dream Sock users in the U.S. once they are launched. A launch date has not been announced.
In 2021, the Lehi-based company temporarily stopped selling its popular baby monitoring socks after getting a warning from the FDA, prompting an outcry from some parents. The FDA warned that Owlet needed to apply for marketing clearance, as the socks were being marketed as medical devices "intended for use in the diagnosis of disease or other conditions or in the cure, mitigation, treatment, or prevention of disease, or to affect the structure or any function of the body."
A petition for the FDA to give the devices clearance garnered nearly 170,000 signatures.
In 2022, Owlet released the sock it currently sells, which tracks a baby's wakings, movement and heart rate. The company last year said the sock met FDA guidance, Medtech Dive reported. The company later added a feature that tracks average blood oxygen levels.
The new FDA clearance means the company will be able to provide more advanced data.
"With this de novo clearance, we are proud to set new standards in at-home infant care, arming parents with reliable real-time information and providing enhanced peace of mind," Workman said.
The Dream Sock was clinically tested in home and hospital environments and deemed as accurate as medical-grade baby monitoring technology. The device also was confirmed to be in compliance with performance and safety standards.
"This new technology will equip caregivers with the right information at the right time, and provide them with confidence and clarity on their baby's well-being," the Owlet press release said.
The company also received clearance earlier this year for its BabySat, a prescription monitoring system for infants with acute or chronic medical conditions. BabySat uses pulse oximetry technology and is used under the supervision of a physician. The Dream Sock is for healthy babies without a prescription.