Estimated read time: 3-4 minutes
This archived news story is available only for your personal, non-commercial use. Information in the story may be outdated or superseded by additional information. Reading or replaying the story in its archived form does not constitute a republication of the story.
WASHINGTON — For the first time, there's a vaccine for pregnant women to protect their babies against respiratory syncytial virus. The U.S. Food and Drug Administration this week approved Abrysvo for use at 32 to 36 weeks of gestation.
The vaccine, made by Pfizer, will protect infants from birth to up to 6 months of age from severe lower respiratory disease symptoms because of RSV, the agency said in announcing the approval, though the efficacy wanes to about 69% by 6 months.
"RSV is a common cause of illness in children and infants are among those at highest risk for severe disease, which can lead to hospitalization," said Dr. Peter Marks, director of the FDA Center for Biologics Evaluation and Research, in the news release. "This approval provides an option for health care providers and pregnant individuals to protect infants from this potentially life-threatening disease."
The Centers for Disease Control and Prevention says nearly all children have had RSV by age 2. It's marked by a runny nose, decreased appetite, coughing, sneezing, fever and wheezing. In the youngest children, irritability, decreased activity and congestion may be the only signs.
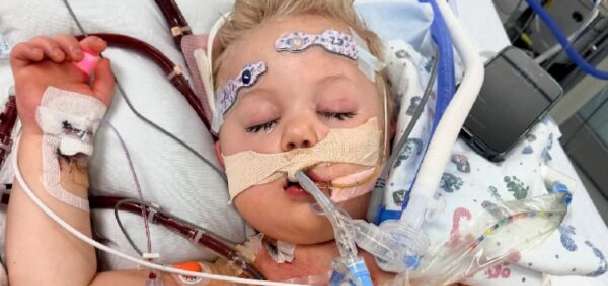
But it can morph into something dangerous in the very young and in older adults. For babies under age 1, RSV can create severe infections including lung inflammation and pneumonia.
Most cases of RSV are mild. But both young children and adults in their 60s and older are at risk of more serious symptoms and potential complications. The CDC says that each year, as many as 300 children under age 5 in the U.S. die. And as many as 160,000 older adults are hospitalized.
Per NBC News, "Last year's RSV season brought a dramatic spike in severe illness that overwhelmed children's hospitals. According to a study published this month, most babies who ended up in the intensive care unit with RSV in late 2022 were previously healthy and born full term."
The injection is given in the muscle. The FDA said the most common side effects for pregnant women are pain at the injection site, muscle pain, fatigue, headache, nausea, joint pain and diarrhea.
The FDA notes a statistically insignificant increase in preterm births among those who received the vaccine in a clinical study, compared to those who received a placebo, but said that "available data are insufficient to establish or exclude a causal relationship" between preterm birth and the vaccine. That contributed to not approving its use before 32 weeks gestation and the prescribing label contains a warning not to administer it before then.
Pfizer must also conduct post-marketing studies to assess the risk of preterm birth and also whether the vaccine contributes to hypertensive disorders of pregnancy such as pre-eclampsia, the FDA said.
NBC notes that expectant parents and those with babies now have two ways to protect them from RSV. Last year, there was none.
Besides the vaccine, an antibody injection is also available, though officials note that the vaccine might work better because of how it prompts an immune response.
"The antibody injection, called Beyfortus, is CDC-recommended for babies up to 8 months old who are born during or entering their first RSV season, which typically starts around October. It's also recommended for infants 8 to 19 months old who are at increased risk of severe disease and entering their second season," NBC reported.