Estimated read time: 5-6 minutes
This archived news story is available only for your personal, non-commercial use. Information in the story may be outdated or superseded by additional information. Reading or replaying the story in its archived form does not constitute a republication of the story.
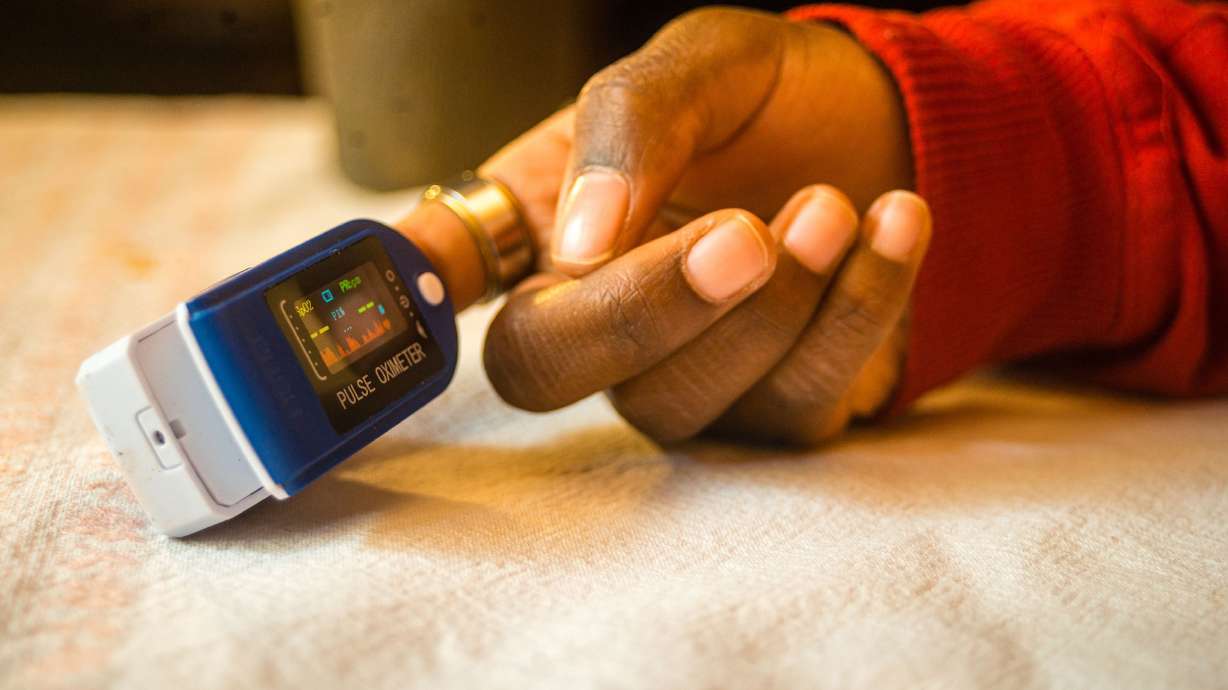
WASHINGTON — The longstanding problem of pulse oximeters providing less-accurate readings for people with dark skin tones got another look Friday from a panel of experts for the Food and Drug Administration.
Pulse oximeters – available both over-the-counter and by prescription – are fingertip clamps that send light beams through your finger to estimate the oxygen saturation of your blood and your pulse rate. Ongoing research has found that if these electronic devices are not calibrated for darker skin tones, the pigmentation could affect how that light is absorbed by their sensors, leading to inaccurate oxygen readings.
Because the general public uses these devices to check their oxygen levels, the FDA panel focused on how to ensure that pulse oximeters are performing accurately for all skin tones before they hit the market.
"There's no question that more diversity needs to be a part of whatever new requirements that they would issue," Dr. Jeffrey Feldman, a member of the panel as well as the American Society of Anesthesiologists' Committee on Equipment and Facilities, said just after the meeting adjourned.
Clinical studies should also evaluate how pulse oximeters perform over time for different skin tones, such as how long it takes for the device to provide a steady, accurate reading, panel member Dr. Tamorah Lewis, a physician scientist and the division head for clinical pharmacology and toxicology at SickKids in Toronto, said in the meeting.
"This technology has and continues to save lives on a daily basis in this country," Feldman said. "It needs to be improved. We need to look at health disparities, and we need to do better," he said after the meeting. "But we also need to recognize how valuable this technology is for patients every day, at home and in the hospital."
'The machines tend to lose her reading for no reason'
The discussions in Friday's meeting were intended to help inform the FDA's ongoing evaluation of pulse oximeters, as the Anesthesiology and Respiratory Therapy Devices Panel assessed ways to improve the quality of premarket studies and heard from patients, researchers and developers of medical devices.
Edward McClure, who was diagnosed in 2013 with the lung disease emphysema, a form of chronic obstructive pulmonary disease or COPD, said he relies on pulse oximeter devices to check his blood oxygen levels because he has been prescribed oxygen therapy. But his readings have not always been accurate, he said, and he suspects that might be due to his skin color.
"Sometimes, when I get a reading, the pulse rate might say 27, and the OT rating might say 90 — and then I know that is not correct, because I know my pulse was not 27," McClure, who is associated with the nonprofit Right2Breathe, told the panel.
"Then I'll do it again, and it might be closer to what I feel is right, but sometimes I have to do it up to three times where I'm convinced that this is the right reading," he said. "These pulse oximeters don't always read accurately for people who have melanated skin or heavily melanated skin like myself."
Ryan Jolly's daughter requires advanced medical care at home, and Jolly uses a pulse oximeter to monitor her oxygen levels while she sleeps.
"We've been using pulse oximeters with her in our home for 10 years," Jolly, who is associated with the International Children's Advisory Network, said in the meeting.
Jolly, who is white, said she noticed that the pulse oximeter readings were much more consistent and accurate for her son, whom she described as being "much paler" than her daughter, who is African American.
"What we see with my daughter when I talk about consistency with a darker skin tone is that the machines tend to lose her reading for no reason that we can detect," Jolly said. "She might be wearing it for hours at a time and we're getting good readings and everything is fine, and then no one moves, nothing happens, and suddenly we're getting an alarm message."
Jolly needs the pulse oximeter readings to be consistent over time so she can continuously monitor her daughter's well-being. McClure needs his pulse oximeter to provide accurate measures quickly so he knows how best to react to his health needs in the moment.
Even with different medical demands, they face the same pulse oximeter shortcomings.
'We need to address the root and work harder'
The pulse oximeter was invented in 1974, and a growing body of research — dating to the 1980s — shows that flawed readings among Black and brown patients can be a real and life-threatening issue in medical care.
"Studies have shown that pulse oximeters are three times more likely to provide misleading readings for patients with darker skin pigmentations, leading to missed critical diagnoses of low blood oxygen levels," Dr. Jesse Ehrenfeld, president of the American Medical Association, said in Friday's meeting.
These devices became more widely used during the COVID-19 pandemic, putting a spotlight on the racial disparities in the accuracy of pulse oximeter readings, said Dr. Dionne Ibekie, an anesthesiologist in central Illinois who is not involved with the FDA panel meeting.
"I will admit, as an anesthesiologist, I and many of my medical colleagues were minimally aware of this discrepancy. It wasn't something really emphasized in training or medical school. So I was shocked, but not surprised to learn about this pulse ox discrepancy," Ibekie, who has talked about racial disparities in medicine on her podcast "The Ivy Drip," wrote in an email.
She was not surprised, she said, because these devices — similar to many standards of care that are developed in medicine — are based on research that historically did not involve diverse groups of people.
"That standard is then applied to all people as a one-size-fits-all, but time and again, we have seen in medicine that this approach leads to poor outcomes for certain groups, especially Black patients," Ibekie said.
"We need to address the root and work harder to conduct research with patients that represent our populations as a whole," she said. "I'm happy the FDA is having a panel because my hope is that it leads to new practice guidelines and the development of new devices that are better suited for all patient groups."