Estimated read time: 7-8 minutes
This archived news story is available only for your personal, non-commercial use. Information in the story may be outdated or superseded by additional information. Reading or replaying the story in its archived form does not constitute a republication of the story.
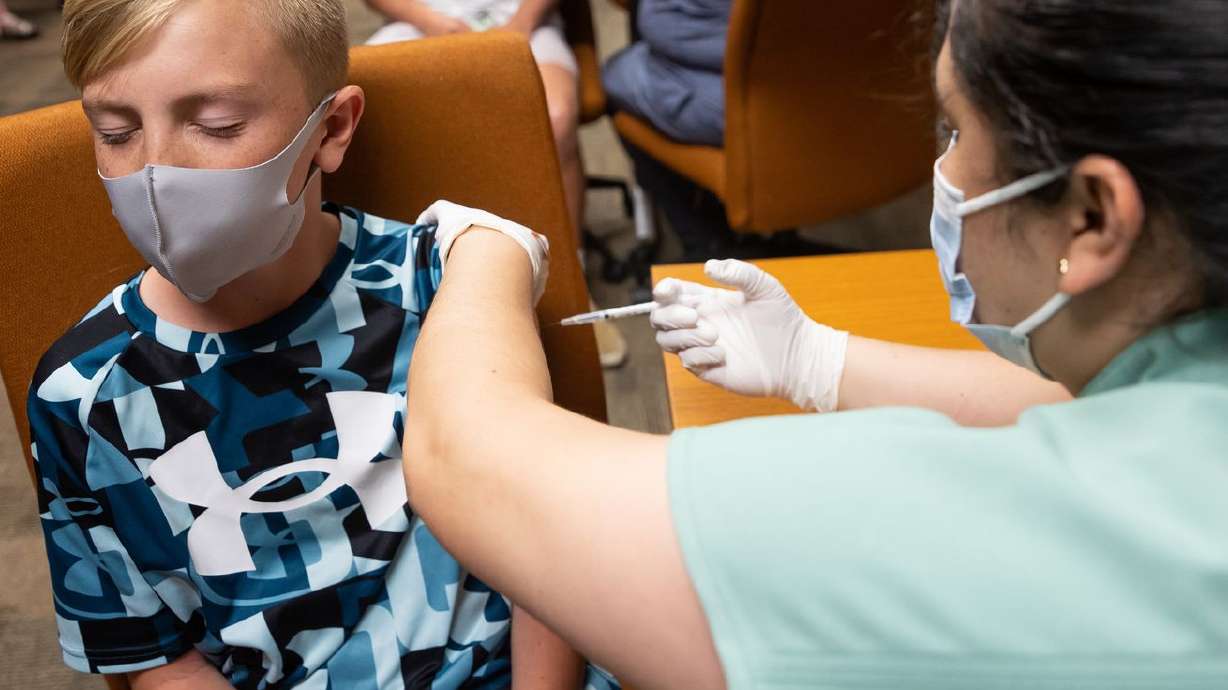
SALT LAKE CITY — Children as young as 5 years old may be able to be vaccinated against COVID-19 by Halloween, now that Pfizer and BioTech are reporting lower doses of their vaccine proved safe while producing a "robust" antibody response in that age group.
The findings announced by the companies earlier this week still must be submitted to the U.S. Food and Drug Administration, which will decide whether to amend the emergency use order allowing adolescents 12 to 15 years old to receive the vaccine to include children 5 to 11 years old.
While the data shared to date appears to be good news for parents anxious to protect their younger children from the deadly virus, experts are waiting to see the details of the latest clinical trial that involved some 2,300 children aged 5-11.
"A press release is just a press release, and we want to see the rest of the data. But hopefully that is going to happen very soon, and hopefully a good, careful look at the full data package will be every bit as encouraging as what they put out in the press release," Dr. Andy Pavia told reporters during a recent virtual news conference.
Pavia, chief of the division of pediatric infectious diseases at University of Utah Health and director of hospital epidemiology at Intermountain Primary Children's Hospital in Salt Lake City, said "that's really the point at which we can say, 'Yes, this is looking great. We're excited about giving it to our children.'"
How serious is COVID-19 for kids?
Lately, there are typically eight to 10 children hospitalized at Primary Children's with COVID-19, Pavia said, "much more than we saw at any time in the past year. I think that reflects both the spread among children that we're seeing this year and the increased infectiousness of delta," the highly contagious virus variant.
School-aged children also account for about 1 of every 4 new cases of the virus in Utah during the current surge, he said, a number that's likely higher since many parents aren't testing their children for the virus because they're worried about having to keep them out of school.
There have been nearly 60,000 cases of the virus in Utahns aged 14 and under, accounting for 12% of all cases in the state, according to the Utah Department of Health. Nearly 500 have been sick enough to be hospitalized, and two Salt Lake County youths have died from the disease, including an unvaccinated teen.
What parents need to do
Deciding whether to vaccinate children against COVID-19 means evaluating the risks involved, Pavia said. Children do get sick enough to be hospitalized or die, but even with milder cases, they miss school and face the possibility of dealing with what's known as long COVID-19 — fatigue, fogginess and other symptoms that linger.
"You have to balance those risks, which people don't always appreciate fully," he said, against the potential risks of the shots that so far, "have proved to be about as safe as any other vaccine that we use." But Pavia said in 5- to 11-year-olds, the study wasn't large enough to know about what he called rarer side effects.
That information will come as the vaccine rolls out in the younger group, he said, adding that if his own children were 5 to 11 years old, they would be at the front of the line for the shots the first day they were available — if they hadn't already been enrolled in a clinical trial.
"What I would say is if your child attends school in Utah, they're at pretty high risk of getting COVID, and they're at pretty high risk of complications," Pavia warned. However, he said, "if they're staying home, if they are in a state where there is universal masking and very low rates of infection, their risk is lower."
For those children at lower risk, the doctor said parents "might want to wait a little bit longer until we know more about rare or minor safety effects." The best source of information for parents, Pavia said, is a family pediatrician or other health care provider.
The bottom line for him, though, is the risk presented by COVID-19 is large while the risk from the vaccine "is almost certainly much, much smaller."
Related:
Will the vaccine really be available by Halloween?
Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases and President Joe Biden's chief medical adviser, has said there is a good chance the shots will be approved for children before they head out trick-or-treating.
FDA officials promised earlier this month to "carefully, thoroughly and independently examine the data to evaluate benefits and risks and be prepared to complete its review as quickly as possible, likely in a matter of weeks rather than months."
But in the same statement, Dr. Janet Woodcock, the acting FDA commissioner, and Dr. Peter Marks, director of the FDA's Center for Biologics Evaluation and Research, also said, "Just like every vaccine decision we've made during this pandemic, our evaluation of data on the use of COVID-19 vaccines in children will not cut any corners."
Pavia said in the past, similar decisions came within weeks of the request being submitted, so the end of October or early November might be when authorization could be anticipated. But he also acknowledged that's just "crystal ball gazing."
After FDA approval, the U.S. Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices must meet to set clinical recommendations. That typically takes only a day or two.
And once the federal government gives the go-ahead, Pavia said he expects the shots to be given to children in the same places adolescents, teens and adults are getting them, including doctors' offices, clinics and pharmacies.
Parents planning ahead for the holidays should realize it takes five weeks from the first dose to be fully immunized. In addition to the three-week wait between the two shots, it takes another two weeks after receiving the final dose before someone is considered fully immunized against the virus.
How the vaccine was tested
The trial tested two doses of the vaccine administered 21 days apart, the same regime currently given to those 12 and older, but the doses were one-third smaller than the standard 30 micrograms. Still, the immune response generated appeared to be equivalent to larger doses in teens.
That's all the companies had to show since the vaccines had been proven effective in stopping COVID-19 infections in studies of older groups, including a trial of 44,000 adults, USA Today reported. Trials are currently underway for children 2 to 5 years old, and 6 months to age 2.
Pfizer and BioTech said the children involved in the studies of the three age groups are from more than 90 locations in the United States, Finland, Poland and Spain, and some had previously had COVID-19, according to USA Today.
The other two coronavirus vaccines approved for use in the United States, the two-dose Moderna and the single-dose Johnson & Johnson, are also being studied in children. Pfizer shots are the only COVID-19 vaccine approved for adolescents and teenagers,
What about 'off label' shots now for children under 12?
That question came up last month, when the Pfizer vaccine was fully approved by the FDA, paving the way for the shots to be prescribed "off-label" for different age groups, conditions or other indications than spelled out by authorities.
But experts say that's not a good idea and have advised waiting until federal authorities have signed off on safety concerns and weighed in on issues like what the proper dosage is for younger children. Pfizer shots are available under an emergency use authorization for those 12 to 16 years old.
Related:
The Utah Department of Health on COVID-19 vaccines for kids
"There is a common misunderstanding that children do not get COVID-19 or are not at risk for severe illness from the virus. However, some children do get sick enough to require treatment in the hospital. There is much we still don't know about how COVID-19 will continue to impact children long-term," the department said in a statement.
"COVID-19 is far more dangerous than any potential risks from getting a vaccine. Children suffer from serious, potentially long-lasting side effects at rates similar to adults, even when they never had symptoms or had only mild symptoms at the time of their infection. Many children continue to suffer with fatigue, headaches, abdominal pain, muscle and joint pain, and difficulty with memory and processing information," the statement continued.
"The Utah Department of Health is eagerly awaiting further recommendations from the FDA and CDC in vaccinating children younger than 12. If you have young children, talk to your health care provider about the best ways to protect them until a vaccine is available."