Estimated read time: 6-7 minutes
This archived news story is available only for your personal, non-commercial use. Information in the story may be outdated or superseded by additional information. Reading or replaying the story in its archived form does not constitute a republication of the story.
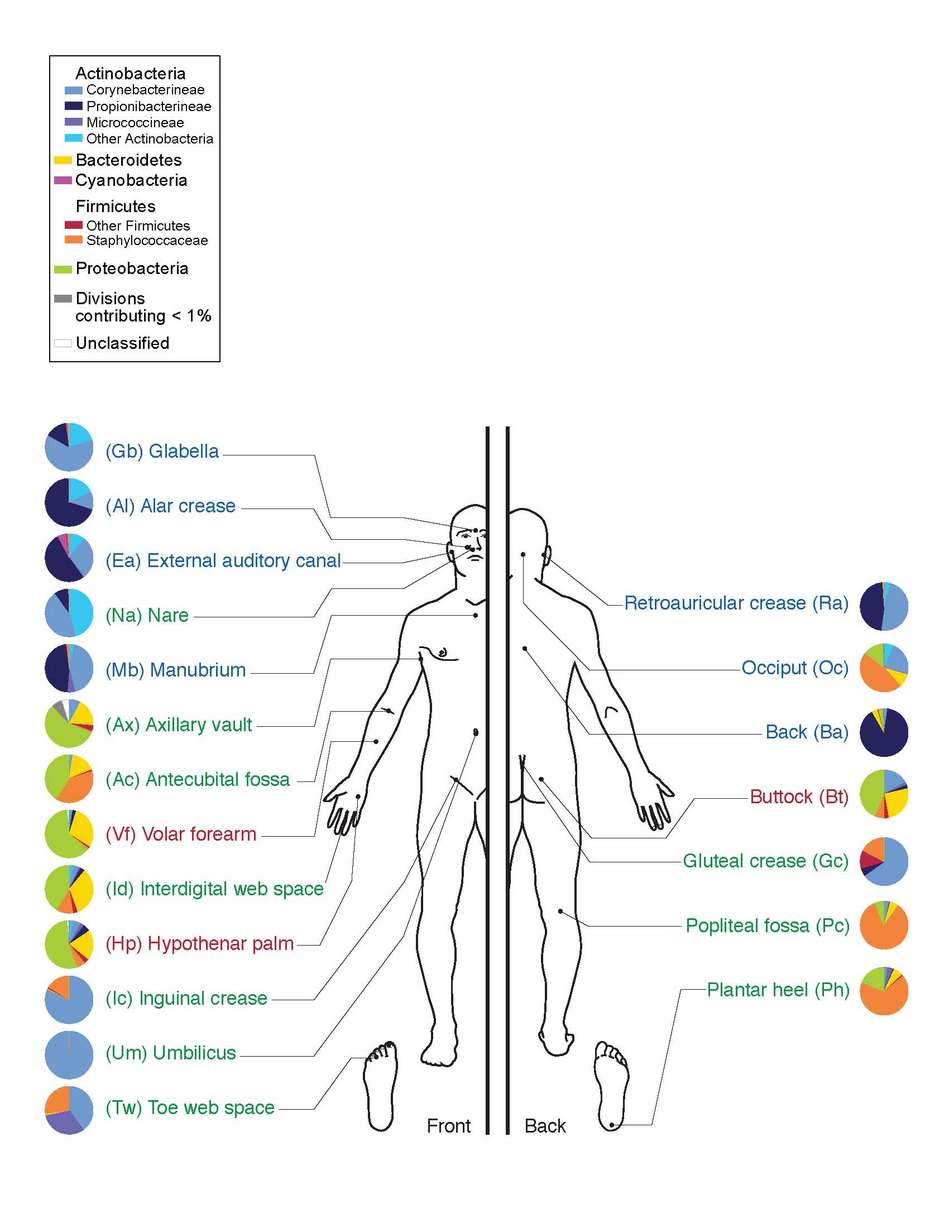
Every human hosts trillions of bacteria and other microorganisms, deriving from more than 1,000 different species of microbes. In fact, inside the human body, bacteria outnumber your own cells 10 to 1. To think of it another way, the human genome contains 20,000 to 25,000 of its own genes. The genes contained in all the bacteria that you host outnumber your own genes by 150 times. Collectively, the interactions of this community of human and bacterial cells is known as the microbiome — also called the bio-zhome.
It has only been in the last ten years that biologists and physiologists have realized just how complex a community it is, and that the human body isn’t the self-sufficient, autonomous organism we once thought it was. In fact, biologists say the interaction between the human body and the bacteria it hosts is best described as an ecosystem.
Inside the human microbiome, biologists see complex, stable, conserved and mutualistic relationships, says Martin Blaser, a professor of internal medicine and microbiology at New York University, with varying levels of metabolic cooperation happening. For instance, some of the bacteria that humans rely on to break down food and make vitamins get their energy from the waste products of other bacteria.
Most of the bacteria found inside humans are friendly, beneficial bacteria carrying out functions that the human body isn’t capable of doing by itself. In fact, the human body is highly reliant of friendly bacteria to function. Most of the enzymes needed to break down food, and the nutrients needed to power and repair our tissues and the bacteria inside us manufacture organs, writes Jennifer Ackerman in the June 2012 issue of “Scientific American.”
For instance, humans get a lot of their energy from complex carbohydrates — things such as potatoes, apples and wheat germs, just to name a few. But, the human body doesn’t make the enzymes necessary to break down complex carbohydrates. This job is performed by bacteroides thetaiotaomicron. Bacteroides makes 260 kinds of enzymes that break down the cells of plants that humans consistently eat. Without b. thetaiotaomicron, complex carbohydrates would be indigestible to humans. After b. thetaiotaomicron breaks down the plant’s cells, it ferments them, generating short-chain fatty acids that the human genome can digest.
Some other examples: About 75 percent of humans host a microorganism called bacteroides fragilis which helps hold the immune system in check by producing a protein that restrains T-cells. When T-cells get too high, it can lead to inflammatory and autoimmune diseases.
Human tissue requires vitamin B-12 for cellular energy, DNA synthesis and the manufacture of fatty acids. This, too, is something that the human body can’t do on its own. Only bacteria make the enzyme needed to make vitamin B-12.
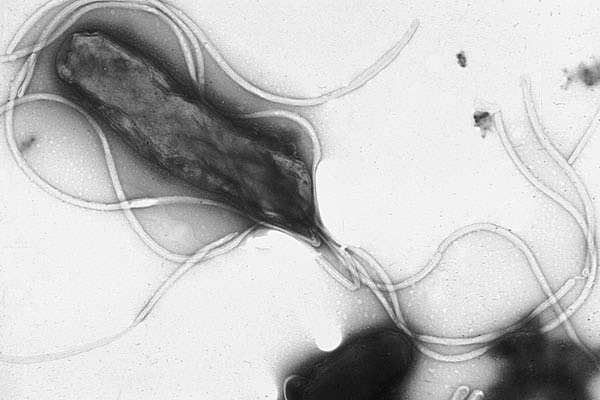
But, the bacteria in the microbiome jungle have some needs and cravings of their own, and they will hijack our own neurology to make this happen. This often makes the human body behave in ways that benefit the bacteria. For example, the human stomach contains bacteria called *helicobacter pylori*. This bacterium regulates the amount of stomach acid that the stomach produces. In addition, h. pylorus produces two hormones directly involved with appetite. The first is the hormone ghrelin, which signals to the brain that the stomach is empty and the body needs to eat. According to Blaser, who has studied h. pylori for the past 25 years, when you wake up in the morning and you feel hungry, it’s because your ghrelin levels are high.
When an h. pylori sense that there is too much acid in the stomach, it creates the hormone ghrelin, which then travels to the human’s brain. The brain simply interprets this increase of ghrelin as hunger, and it eats. After eating, the acidity in the stomach drops and h. pylori stops making ghrelin, which the brain interprets as, “I’m full. I’ll stop eating.”
But, as anyone who has ever been sick knows, not all bacteria that enter the human body are good. The body walks a fine balance between good bacteria and bad bacteria. So how does the body differentiate between good and bad bacteria? Well, that is the question of the century, and, to state it simply, biologists and physiologists still don’t know. But, emerging evidence suggests that the job of some of the bacteria found in the microbiome ecosystem is to fight invading, bad bacteria, Blaser said.
By age 15, most children in the U.S. have had multiple rounds of antibiotics, for things such strep throat and ear infections, Ackerman said. While the antibiotics kill the pathogenic bacteria, it also kills off many of the beneficial bacteria, and this can have consequences on the health of the human.
For example, let’s go back to the appetite-regulating hormone ghrelin, which is produced by h. pylori. Two or three generations ago, more than 80 percent of Americans hosted h. pylori in their stomachs, according to Blaser. But now, less than 6 percent of American kids tested positive for it, due to all the antibiotics they have consumed in their lives. What this means, says Blaser, is that we have a whole generation of children who are growing up without h. pylori to regulate their gastric ghrelin. This lack of h. pylori may be a contributing factor in the rise in childhood obesity, because after they eat there is no ghrelin telling the brain to stop eating.
No two people share the same microbial makeup, but most people share a core complement of helpful bacteria, says Ackerman.
Each human has a community of microbes unique to them that they have acquired on their journey through life. Because the womb doesn’t contain bacteria, newborns start their life microbe free, but they quickly begin acquiring them from breastfeeding, handling by parents and contact with their environment. Once inside the body, these microbes quickly multiply. “By late infancy their bodies support one of the most complex ecosystems on the planet,” Ackerman said.
But the complexity of the human microbiome has been reduced in recent generations due to antibiotics, and the over-sterilization of our environment, due to places such as our kitchens and bathrooms being sprayed or wiped down with disinfectants. These are bacteria that would normally find their way into the human microbiome. The good bacteria would be allowed to stay, and the bad bacteria would be killed.
Biologists don’t yet know how the microbiome differentiates between good and bad bacteria, but they do know that some of the friendly bacteria in our microbiome are there just to fight other invading microbes. When the guardian bacteria don’t have other bacteria to fight, they may turn against the cells of the human body and this can lead to autoimmune diseases and allergies.
The long-term effects of all this over-sterilization are still to be determined, but Blaser says, “I am worried.”











